Research Article - Biomedical Research (2017) Volume 28, Issue 2
5α-reductase inhibitors and anti-prostate cancer activities of some synthesized 4`-(aryl)-4-pregneno[3,2-e]pyridinone derivatives
Abd El-Galil E Amr1,2*, Mohamed M Abdalla3, Mohamed MM Hussein4, Hany M Safwat4 and Mohamed H Elgamal21Pharmaceutical Chemistry Department, Drug Exploration & Development Chair (DEDC), College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia
2National Research Center, Dokki, Cairo, Egypt
3Research Unit, Saco Pharm. Co., 6th October City 11632, Egypt
4Pharmaceutical Chemistry Department, Faculty of Pharmacy, Cairo University, Gizza, Egypt
- *Corresponding Author:
- Abd El-Galil E Amr
Department of Pharmaceutical Chemistry
College of Pharmacy
King Saud University
Saudi Arabia
Accepted on June 08, 2016
Abstract
A series of 2`-mercapto-3`-cyano-4`-(aryl)-4-pregneno-[3,2-e]pyridine-20-ones (3a-h) and 1`- thiocarbamoyl -2`-amino-4`-(aryl)-4-pregneno-[3,2-e]pyridine-20-one derivatives (4a-h) were synthesized using progesterone 1 "17-acetyl-1,7,8,10,11,12,13,15,16,17-decahydro-10,13-dimethyl-2Hcyclopenta[ a]phenanthren-3(6H,9H,14H)-one" as starting material. The synthesized compounds were evaluated for 5α-reductase inhibitors and anti-prostate cancer activities compared to Anastrozole® as positive control. Some of the tested compounds exhibited better activities than Anastrozole®. Compounds 3a-h and 4a-h showed potent 5α-reductase inhibitors and anti-prostate cancer activities than Anastrozole®.
Keywords
Progesterone, pregneno[3,2-e]pyridinones, 5α-reductase inhibitor, anti-prostate cancer.
Introduction
Pyridine derivatives were reported to possess anticonvulsant [1,2], cardiotonic [3], antihypertensive [4], b-adrenergic blocking activity [5]. In the previous work, we found that certain substituted steroidal derivatives were reported to be associated with anti-arrhythmic [6], reductase inhibitor [7], antiandrogenic [8,9], anti-inflammatory [10], and anti-alzheimer [11,12] activities.
Some of heterocyclic compounds containing nitrogen atom were evaluated and reported as anticancer [13], antiparkinsonian [14], anti-inflammatory agents [15] and antimicrobial [16]. In addition, several of heterocyclic candidates exhibited analgesic [17], anticonvulsant, anti-inflammatory and antimicrobial [18,19], antitumor [20], antipyretics [21], and anti-arrhythmic [22] activities.
Also the synthesis and pharmacological activities of steroidal derivatives exhibited androgen receptor antagonists [23], cytotoxic activities [24], anti-proliferative activities in a human androgen-responsive prostate cancer cell line [25]. In view of these observation, we report synthesis of 4`-(aryl)-4- pregneno[3,2-e]pyridinone derivatives and evaluated as 5α- reductase inhibitors and anti-prostate cancer agents.
Material and Methods
Chemistry
All melting points are uncorrected and were measured using an electrothermal capillary melting point apparatus. The IR spectra were recorded on a Shimadzu FT-IR 8101 PC infrared spectrophotometer. The 1H-NMR spectra were determined with Bruker AM-200 MHz spectrometer. The chemical shifts are expressed on the δ (ppm) scale using TMS as the standard reference. Mass spectra were recorded on Finnigan SSQ operating at 70 ev. Elemental analysis determined on a Perkin Elmer 240 (microanalysis), Microanalysis Center, Cairo University, Cairo, Egypt.
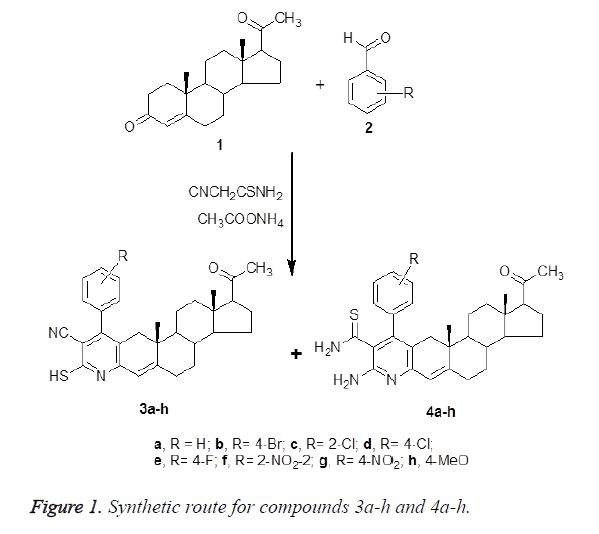
Synthesis of 2`-mercapto-3`-cyano-4`-(aryl)-4- pregneno-[3,2-e]pyridine-20-ones (3a-h) and 1`- thiocarbamoyl -2`-amino-4`-(aryl)-4-pregneno-[3,2- e]pyridine-20-ones (4a-h)
General procedure: To a solution of progesterone 1 (0.314 g, 1 mmol), thiocyanoacetamide (0.100 g, 1.2 mmol), and appropriate aromatic aldehydes, namely, benzaldehyde, 4- bromo-, 2-chloro-, 4-chloro-, 4-flouro-, 2-nitro-, 4-nitro- or 4- methoxybenzaldehyde (2) (1 mmol) in n-butanol (10 mL), ammonium acetate (0.608 g, 8 mmol) was added. The reaction mixture was refluxed for 8 hours with stirring, and evaporated to dryness. The formed residue dissolved 10% hydrochloric acid (100 mL), washed with dichloromethane several time. The aqueous solution was alkalinized with 10% sodium bicarbonate, the resulted precipitate product was filtered off, dried and resorting to flash chromatography technique to separate compounds (3a-h) and (4a-h) by using eluent (benzene : MeOH; 9 : 1, v/v).
2`-Mercapto-3`-cyano-4`-(phenyl)-4-pregneno[3,2- e]pyridine-20-one (3a). Yield 32%, m.p. 255-257°C, [α]25 D= + 79 (c 1, CHCl3); IR (KBr): 3650 (SH), 3055 (CH-Ar), 2900 (CH-aliph), 2150 (CN), 1723 (C=O), 1630 (C=C) cm-1. 1H NMR (CDCl3): δ 0.86 (s, 3H, CH3), 0.99-1.00 (m, 1H, CH), 1.18 (s, 3H, CH3), 1.26-1.35 (m, 4H, 2CH2), 1.42-1.54 (m, 4H, 2CH2), 1.62-1.66 (m, 6H, 3CH2), 1.72-1.85 (m, 1H, CH), 2.17 (s, 3H, COCH3), 2.35-2.40 (m, 1H, CH), 2.55 (m, 1H, CH), 6.10 (s, 1H, SH exchangeable with D2O), 6.24 (s, 1H, CH), 7.22-7.56 (m, 5H, Ar-H); MS (EI): m/z 482 (54%) [M+]. Anal. C31H34N2OS (582.68): Calcd. C, 77.14; H, 7.10; N, 5.80; S, 6.64; found C, 77.10; H, 7.01; N, 5.71; S, 6.55.
2`-Mercapto-3`-cyano-4`-(4-bromophenyl)-4-pregneno[3,2- e]pyridine-20-one (3b). Yield 21%, m.p. 267-269°C, [α]25 D = + 57 (c 1, CHCl3); IR (KBr): 3651 (SH), 3055 (CH-Ar), 2910 (CH-aliph), 2150 (CN), 1725 (C=O), 1630 (C=C) cm-1. 1H NMR (CDCl3): δ 0.89 (s, 3H, CH3), 0.98-1.02 (m, 1H, CH), 1.22 (s, 3H, CH3), 1.28-1.36 (m, 4H, 2CH2), 1.41-1.56 (m, 4H, 2CH2), 1.61-1.65 (m, 6H, 3CH2), 1.72-1.84 (m, 1H, CH), 2.17 (s, 3H, COCH3), 2.32-2.42 (m, 1H, CH), 2.55 (m, 1H, CH), 6.12 (s, 1H, SH exchangeable with D2O), 6.22 (s, 1H, CH), 7.18-7.60 (m, 4H, Ar-H); MS (EI): m/z 561 (45%) [M+]. Anal. C31H33BrN2OS (561.58): Calcd. C, 66.30; H, 5.92; N, 4.99; S, 5.71; found C, 66.15; H, 5.84; N, 4.90; S, 5.60.
2`-Mercapto-3`-cyano-4`-(2-chlorophenyl)-4-pregneno[3,2- e]pyridine-20-one (3c). Yield 22%, m.p. 284-285°C, [α]25 D = + 75 (c 1, CHCl3); IR (KBr): 3650 (SH), 3058 (CH-Ar), 2900 (CH-aliph), 2158 (CN), 1723 (C=O), 1637 (C=C) cm-1. 1H NMR (CDCl3): δ 0.90 (s, 3H, CH3), 0.97-1.05 (m, 1H, CH), 1.21 (s, 3H, CH3), 1.27-1.35 (m, 4H, 2CH2), 1.42-1.57 (m, 4H, 2CH2), 1.60-1.64 (m, 6H, 3CH2), 1.70-1.85 (m, 1H, CH), 2.16 (s, 3H, COCH3), 2.34-2.43 (m, 1H, CH), 2.56 (m, 1H, CH), 6.15 (s, 1H, SH exchangeable with D2O), 6.34 (s, 1H, CH), 7.18-7.60 (m, 4H, Ar-H); MS (EI): m/z 517 (65%) [M+]. Anal. C31H33ClN2OS (517.12): Calcd. C, 72.00; H, 6.43; Cl, 6.86; N, 5.42; S, 6.20; found C, 71.88; H, 6.32; Cl, 6.80; N, 5.33; S, 6.08.
2`-Mercapto-3`-cyano-4`-(4-chlorophenyl)-4-pregneno[3,2- e]pyridine-20-one (3d). Yield 36%, m.p. 269-271°C, [α]25 D = + 91(c 1, CHCl3); IR (KBr): 3651 (SH), 3056 (CH-Ar), 2904 (CH-aliph), 2155 (CN), 1722 (C=O), 1632 (C=C) cm-1. 1H NMR (CDCl3): δ 0.87 (s, 3H, CH3), 0.96-1.00 (m, 1H, CH), 1.18 (s, 3H, CH3), 1.27-1.36 (m, 4H, 2CH2), 1.40-1.57 (m, 4H, 2CH2), 1.62-1.66 (m, 6H, 3CH2), 1.73-1.85 (m, 1H, CH), 2.18 (s, 3H, COCH3), 2.30-2.41 (m, 1H, CH), 2.57 (m, 1H, CH), 6.24 (s, 1H, CH), 6.27 (s, 1H, SH exchangeable with D2O), 7.14-7.64 (m, 4H, Ar-H); MS (EI): m/z 517 (16%) [M+]. Anal. C31H33ClN2OS (517.12): Calcd. C, 72.00; H, 6.43; Cl, 6.86; N, 5.42; S, 6.20; found C, 71.90; H, 6.32; Cl, 6.79; N, 5.34; S, 6.17.
2`-Mercapto-3`-cyano-4`-(4-fluorophenyl)-4-pregneno[3,2- e]pyridine-20-one (3e). Yield 22%, m.p. 253-255°C, [α]25 D = + 22.5(c 1, CHCl3); IR (KBr): 3650 (SH), 3056 (CH-Ar), 2909 (CH-aliph), 2159 (CN), 1723 (C=O), 1630 (C=C) cm-1. 1H NMR (CDCl3): δ 0.86 (s, 3H, CH3), 0.97-1.04 (m, 1H, CH), 1.17 (s, 3H, CH3), 1.26-1.35 (m, 4H, 2CH2), 1.38-1.48 (m, 4H, 2CH2), 1.60-1.64 (m, 6H, 3CH2), 1.70-1.84 (m, 1H, CH), 2.19 (s, 3H, COCH3), 2.31-2.40 (m, 1H, CH), 2.56 (m, 1H, CH), 6.10 (s, 1H, SH exchangeable with D2O), 6.26 (s, 1H, CH), 7.14-7.58 (m, 4H, Ar-H); MS (EI): m/z 500 (18%) [M+]. Anal. C31H33FN2OS (500.67): Calcd. C, 74.37; H, 6.64; N, 5.60; S, 6.40; found C, 74.28; H, 6.55; N, 5.50; S, 6.30.
2`-Mercapto-3`-cyano-4`-(2-nitrophenyl)-4-pregneno[3,2- e]pyridine-20-one (3f). Yield 31%, m.p. 265-267°C, [α]25 D = + 88 (c 1, CHCl3); IR (KBr): 3651 (SH), 3054 (CH-Ar), 2933 (CH-aliph), 2145 (CN), 1734 (C=O), 1641 (C=C) cm-1. 1H NMR (CDCl3): δ 0.85 (s, 3H, CH3), 0.98-1.05 (m, 1H, CH), 1.20 (s, 3H, CH3), 1.27-1.35 (m, 4H, 2CH2), 1.40-1.55 (m, 4H, 2CH2), 1.61-1.66 (m, 6H, 3CH2), 1.71-1.85 (m, 1H, CH), 2.18 (s, 3H, COCH3), 2.32-2.42 (m, 1H, CH), 2.55 (m, 1H, CH), 6.25 (s, 1H, CH), 6.30 (s, 1H, SH exchangeable with D2O), 7.30-7.96 (m, 4H, Ar-H); MS (EI): m/z 528 (32%) [M+]. Anal. C31H33N3O3S (527.68): Calcd. C, 70.56; H, 6.30; N, 7.96; S, 6.08; found C, 70.50; H, 6.22; N, 7.88; S, 6.00.
2`-Mercapto-3`-cyano-4`-(4-nitrophenyl)-4-pregneno[3,2- e]pyridine-20-one (3g). Yield 31%, m.p. 278-280°C, [α]25 D = + 34(c 1, CHCl3); IR (KBr): 3651 (SH), 3058 (CH-Ar), 2914 (CH-aliph), 2158 (CN), 1722 (C=O), 1632 (C=C) cm-1. 1H NMR (CDCl3): δ 0.88 (s, 3H, CH3), 0.99-1.04 (m, 1H, CH), 1.19 (s, 3H, CH3), 1.25-1.33 (m, 4H, 2CH2), 1.38-1.54 (m, 4H, 2CH2), 1.60-1.67 (m, 6H, 3CH2), 1.70-1.86 (m, 1H, CH), 2.17 (s, 3H, COCH3), 2.30-2.40 (m, 1H, CH), 2.58 (m, 1H, CH), 6.22 (s, 1H, CH), 6.27 (s, 1H, SH exchangeable with D2O), 7.28-7.98 (m, 4H, Ar-H); MS (EI): m/z 527 (52%) [M+]. Anal. C31H33N3O3S (527.68): Calcd. C, 70.56; H, 6.30; N, 7.96; S, 6.08; found C, 70.48; H, 6.13; N, 7.87; S, 6.01.
2`-Mercapto-3`-cyano-4`-(4-methoxyphenyl)-4- pregneno[3,2-e]pyridine-20-one (3h). Yield 28%, m.p. 258-260°C, [α]25 D = + 46 (c 1, CHCl3); IR (KBr): 3625 (SH), 3055 (CH-Ar), 2922 (CH-aliph), 2148 (CN), 1734 (C=O), 1628 (C=C) cm-1. 1H NMR (CDCl3): δ 0.87 (s, 3H, CH3), 0.96-1.00 (m, 1H, CH), 1.19 (s, 3H, CH3), 1.26-1.36 (m, 4H, 2CH2), 1.41-1.57 (m, 4H, 2CH2), 1.61-1.65 (m, 6H, 3CH2), 1.72-1.85 (m, 1H, CH), 2.18 (s, 3H, COCH3), 2.31-2.41 (m, 1H, CH), 2.57 (m, 1H, CH), 3.75 (s, 3H, OCH3), 6.10 (s, 1H, SH exchangeable with D2O), 6.24 (s, 1H, CH), 7.15-7.57 (m, 4H, Ar-H); MS (EI): m/z 512 (24%) [M+]. Anal. C32H36N2O2S (512.71): Calcd. C, 74.96; H, 7.08; N, 5.46; S, 6.25; found C, 74.87; H, 7.00; N, 5.33; S, 6.18.
1`-Thiocarbamoyl-2`-amino-4`-(phenyl)-4-pregneno[3,2- e]pyridine-20-one (4a). Yield 28%, m.p. 232-234°C, [α]25 D = + 46 (c 1, CHCl3); IR (KBr): 3505 (NH2), 3021 (CH-Ar), 2950 (CH-aliph), 1730 (C=O), 1628 (C=C) cm-1. 1H NMR (CDCl3): δ 0.86 (s, 3H, CH3), 0.97-1.01 (m, 1H, CH), 1.18 (s, 3H, CH3), 1.26-1.34 (m, 4H, 2CH2), 1.42-1.55 (m, 4H, 2CH2), 1.60-1.65 (m, 6H, 3CH2), 1.72-1.87 (m, 1H, CH), 2.18 (s, 3H, COCH3), 2.36-2.41 (m, 1H, CH), 2.54 (m, 1H, CH), 4.29 (s, 1H, NH exchangeable with D2O), 6.23 (s, 1H, CH), 7.21-7.54 (m, 5H, Ar-H), 8.21 (s, 2H, NH2 exchangeable with D2O); MS (EI): m/z 500 (32%) [M+]. Anal. C31H37N3OS (499.71): Calcd. C, 74.51; H, 7.46; N, 8.41; S, 6.42; found C, 74.42; H, 7.34; N, 8.32; S, 6.33.
1`-Thiocarbamoyl-2`-amino-4`-(4-bromophenyl)-4- pregneno[3,2-e]pyridine-20-one (4b). Yield 25%, m.p. 256-258°C, [α]25 D = + 123 (c 1, CHCl3); IR (KBr): 3509 (NH2), 3021 (CH-Ar), 2959 (CH-aliph), 1730 (C=O), 1627 (C=C) cm-1. 1H NMR (CDCl3): δ 0.88 (s, 3H, CH3), 0.98-1.00 (m, 1H, CH), 1.16 (s, 3H, CH3), 1.24-1.32 (m, 4H, 2CH2), 1.41-1.55 (m, 4H, 2CH2), 1.61-1.66 (m, 6H, 3CH2), 1.70-1.85 (m, 1H, CH), 2.16 (s, 3H, COCH3), 2.35-2.40 (m, 1H, CH), 2.55 (m, 1H, CH), 4.30 (s, 1H, NH exchangeable with D2O), 6.34 (s, 1H, CH), 7.24-7.56 (m, 4H, Ar-H), 8.18 (s, 2H, NH2 exchangeable with D2O); MS (EI): m/z 578 (42%) [M+]. Anal. C31H36BrN3OS (578.61): Calcd. C, 64.35; H, 6.27; N, 7.26; S, 5.54; found C, 64.22; H, 6.20; N, 7.20; S, 5.46.
1`-Thiocarbamoyl-2`-amino-4`-(2-chlorophenyl)-4- pregneno[3,2-e]pyridine-20-one (4c). Yield 29%, m.p. 288-290°C, [α]25 D = + 44 (c 1, CHCl3); IR (KBr): 3518 (NH2), 3021 (CH-Ar), 2967 (CH-aliph), 1745 (C=O), 1631 (C=C) cm-1. 1H NMR (CDCl3): δ 0.89 (s, 3H, CH3), 0.98-1.05 (m, 1H, CH), 1.18 (s, 3H, CH3), 1.25-1.34 (m, 4H, 2CH2), 1.40-1.54 (m, 4H, 2CH2), 1.62-1.65 (m, 6H, 3CH2), 1.71-1.86 (m, 1H, CH), 2.17 (s, 3H, COCH3), 2.32-2.38 (m, 1H, CH), 2.53 (m, 1H, CH), 4.67 (s, 2H, NH2 exchangeable with D2O), 6.34 (s, 1H, CH), 7.18-7.565(m, 4H, Ar-H), 8.16 (s, 2H, NH2 exchangeable with D2O); MS (EI): m/z 534 (12%) [M+]. Anal. C31H36ClN3OS (534.16): Calcd. C, 69.70; H, 6.79; Cl, 6.64; N, 7.87; S, 6.00; found C, 69.59; H, 6.71; Cl, 6.54; N, 7.82; S, 5.89.
1`-Thiocarbamoyl-2`-amino-4`-(4-chlorophenyl)-4- pregneno[3,2-e]pyridine-20-one (4d). Yield 17%, m.p. 345-247°C, [α]25 D = + 78 (c 1, CHCl3); IR (KBr): 3522 NH2), 3034 (CH-Ar), 2980 (CH-aliph), 1740 (C=O), 1631 (C=C) cm-1. 1H NMR (CDCl3): δ 0.87 (s, 3H, CH3), 0.96-1.00 (m, 1H, CH), 1.18 (s, 3H, CH3), 1.27-1.36 (m, 4H, 2CH2), 1.40-1.57 (m, 4H, 2CH2), 1.62-1.66 (m, 6H, 3CH2), 1.73-1.85 (m, 1H, CH), 2.18 (s, 3H, COCH3), 2.36-2.41 (m, 1H, CH), 2.57 (m, 1H, CH), 4.80 (s, 2H, NH2 exchangeable with D2O), 6.24 (s, 1H, CH), 7.28-7.60 (m, 4H, Ar-H), 8.36 (s, 2H, NH2 exchangeable with D2O); MS (EI): m/z 534 (52%) [M+]. Anal. C31H36ClN3OS (534.16): Calcd. C, 69.70; H, 6.79; Cl, 6.64; N, 7.87; S, 6.00; found C, 69.58; H, 6.70; Cl, 6.55; N, 7.80; S, 5.88.
1`-Thiocarbamoyl-2`-amino-4`-(4-fluorophenyl)-4- pregneno[3,2-e]pyridine-20-one (4e). Yield 19%, m.p. 276-278°C, [α]25 D = + 69 (c 1, CHCl3); IR (KBr): 3524 (NH2), 3030 (CH-Ar), 2977 (CH-aliph), 1738 (C=O), 1631 (C=C) cm-1. 1H NMR (CDCl3): δ 0.88 (s, 3H, CH3), 0.97-1.04 (m, 1H, CH), 1.17 (s, 3H, CH3), 1.26-1.35 (m, 4H, 2CH2), 1.38-1.48 (m, 4H, 2CH2), 1.60-1.64 (m, 6H, 3CH2), 1.70-1.84 (m, 1H, CH), 2.19 (s, 3H, COCH3), 2.34-2.40 (m, 1H, CH), 2.56 (m, 1H, CH), 4.93 (s, 2H, NH2 exchangeable with D2O), 6.26 (s, 1H, CH), 7.15-7.62 (m, 4H, Ar-H), 8.35 (s, 2H, NH2 exchangeable with D2O); MS (EI): m/z 517 (24%) [M+]. Anal. C31H36FN3OS (517.7): Calcd. C, 71.92; H, 7.01; N, 8.12; S, 6.19; found C, 71.80; H, 6.96; N, 8.00; S, 6.10.
1`-Thiocarbamoyl-2`-amino-4`-(2-nitrophenyl)-4- pregneno[3,2-e]pyridine-20-one (4f). Yield 31%, m.p. 236-238°C, [α]25 D = + 150 (c 1, CHCl3); IR (KBr): 3509 (NH2), 3021 (CH-Ar), 2949 (CH-aliph), 1730 (C=O), 1634 (C=C) cm-1. 1H NMR (CDCl3): δ 0.91 (s, 3H, CH3), 0.98-1.05 (m, 1H, CH), 1.20 (s, 3H, CH3), 1.27-1.35 (m, 4H, 2CH2), 1.40-1.55 (m, 4H, 2CH2), 1.61-1.66 (m, 6H, 3CH2), 1.71-1.85 (m, 1H, CH), 2.18 (s, 3H, COCH3), 2.36-2.42 (m, 1H, CH), 2.55 (m, 1H, CH), 4.70 (s, 2H, NH2 exchangeable with D2O), 6.25 (s, 1H, CH), 7.29-7.92 (m, 4H, Ar-H), 8.32 (s, 2H, NH2 exchangeable with D2O); MS (EI): m/z 544 (100%) [M+]. Anal. C31H36N4O3S (544.71): Calcd. C, 68.35; H, 6.66; N, 10.29; S, 5.89; found C, 68.13; H, 6.58; N, 10.18; S, 5.79.
1`-Thiocarbamoyl-2`-amino-4`-(4-nitrophenyl)-4- pregneno[3,2-e]pyridine-20-one (4g). Yield 26%, m.p. 358-360°C, [α]25 D = + 179(c 1, CHCl3); IR (KBr): 3520 (NH2), 3033 (CH-Ar), 2956 (CH-aliph), 1742 (C=O), 1646 (C=C) cm-1. 1H NMR (CDCl3): δ 0.89 (s, 3H, CH3), 0.99-1.03 (m, 1H, CH), 1.18 (s, 3H, CH3), 1.28-1.33 (m, 4H, 2CH2), 1.39-1.55 (m, 4H, 2CH2), 1.60-1.65 (m, 6H, 3CH2), 1.70-1.85 (m, 1H, CH), 2.17 (s, 3H, COCH3), 2.35-2.40 (m, 1H, CH), 2.58 (m, 1H, CH), 4.68 (s, 2H, NH2 exchangeable with D2O), 6.22 (s, 1H, CH), 7.32-7.95 (m, 4H, Ar-H), 8.45 (s, 2H, NH2 exchangeable with D2O); MS (EI): m/z 544 (100%) [M+]. Anal. C31H36N4O3S (544.71): Calcd. C, 68.35; H, 6.66; N, 10.29; S, 5.89; found C, 68.27; H, 6.60; N, 10.20; S, 5.80.
1`-Thiocarbamoyl-2`-amino-4`-(4-methoxyphenyl)-4- pregneno[3,2-e]pyridine-20-one (4h). Yield 18%, m.p. 291-293°C, [α]25 D = + 100 (c 1, CHCl3); IR (KBr): 3515 (NH2), 3031 (CH-Ar), 2960 (CH-aliph), 1740 (C=O), 1630 (C=C) cm-1. 1H NMR (CDCl3): δ 0.87 (s, 3H, CH3), 0.94-1.00 (m, 1H, CH), 1.21 (s, 3H, CH3), 1.26-1.36 (m, 4H, 2CH2), 1.41-1.57 (m, 4H, 2CH2), 1.61-1.65 (m, 6H, 3CH2), 1.72-1.85 (m, 1H, CH), 2.18 (s, 3H, COCH3), 2.33-2.41 (m, 1H, CH), 2.57 (m, 1H, CH), 3.72 (s, 3H, OCH3), 4.48 (s, 1H, NH exchangeable with D2O), 6.24 (s, 1H, CH), 7.25-7.55 (m, 4H, Ar-H), 8.27 (s, 2H, NH2 exchangeable with D2O); MS (EI): m/z 529 (40%) [M+]. Anal. C32H39N3O2S (529.74): Calcd. C, 72.55; H, 7.42; N, 7.93; S, 6.05; found C, 72.44; H, 7.35; N, 7.86; S, 6.00.
5α-Reductase inhibitors
Treatment of animals: Animals were obtained from the animal house colony of the NRC Cairo Egypt. All animals were allowed free access to water and were kept on a constant standard diet. 19 Groups, each of 12 male Sprague-Dawley rats in the postnatal third days, were treated subcutaneously with the 5α-reductase inhibitor (tested compound or reference standard).
The tested compounds were suspended in 5% Tween 80 in water. The vehicle was used for both standard and negative control group, beginning on the postnatal third day until the age of seven weeks. 18 Groups were used to test the activities, of which one was used as the positive control for anastrozole and another served as the negative control group. After scarifying, blood was withdrawn for testosterone and dihydrotestosterone (DHT) determination [26]. Moreover, intraprostatic concentrations of testosterone and DHT were determined [27]. The biological experiments were performed according to the official standards.
Anti-prostate cancer screening anti-androgenic bioassay in human prostate cancer cells
Human prostate cancer LNCaP and PC-3 cells were maintained in RPMI medium and Dulbecco's minimum essential medium (DMEM), respectively. Both media were supplemented with penicillin (25 units/mL), streptomycin (25 μg/mL), and 10% fetal calf serum. For the androgen receptor transactivation assay, an androgen-dependent reporter gene transcription test was employed as the primary screening for potential antiandrogen identification. This assay was first performed in LNCaP cells, which express a clinically relevant mutant AR. Once anti-androgenic activity was detected in the LNCaP AR transactivation assay, compounds were re-examined for their potential activity against wild type AR. Wild type AR transactivation assay was performed in PC-3 host cells, which lack an endogenous, functional AR.
The method and conditions of cell and gene transfection have been described previously. In brief, cells were plated in 24-well tissue culture dishes for 24 (PC-3 cells) or 48 (LNCaP cells) h prior to transfection. Subsequently, LNCaP cells were transfected with a reporter gene, MMTV-luciferase, which contains MMTV-LTR promoter and androgen receptor binding element, and PRL-SV40, which served as an internal control for transfection efficiency. PC-3 cells were transfected with a wild type AR expression plasmid, pSG5AR, in addition to the above-mentioned MMTV-luciferase reporter gene and PRL-SV40 internal control. SuperFect (Qiagen, Chatsworth, CA) was employed as the transfection reagent following manufacturer's recommendations.
At the end of a five-hour transfection, the medium was changed to DMEM or RPMI supplemented with 10% charcoal dextran-stripped, i.e., androgen-depleted, serum. After 24 h, the cells were treated with 1 nM of DHT and/or test compounds at the designated concentration for another 24 h. The cells were harvested for luciferase activity assay using Dual Luciferase Assay System (Promega, Madison, WI). The derived data were expressed as relative luciferase activity normalized to the internal luciferase control. Cells cultured in medium containing DHT (androgen), as a control, induced a marked reporter gene expression. Test compounds capable of significantly suppressing this DHT-induced reporter gene expression were identified as potential antiandrogens [27,28].
Results and Discussion
Chemistry
A series of 2`-mercapto-3`-cyano-4`-(aryl)-4-pregneno-[3,2- e]pyridine-20-one (3a-h) and 1`-thiocarbamoyl -2`-amino-4`- (aryl)-4-pregneno-[3,2-e]pyridine-20-one derivatives (3a-h) were synthesized using progesterone (1) as starting materials. Treatment of 1 with thiocyanoa-cetamide and appropriate aromatic aldehydes, namely, benzaldehyde, 4-bromo-, 2- chloro-, 4-chloro-, 4-flouro-, 2-nitro-, 4-nitro- or 4- methoxybenzaldehyde (2) in the presence of ammonium acetate in n-butanol afforded the corresponding 4- pregneno[3,2-e]pyridine-20-one derivatives 3a-h and 4a-h as a mixture which was chromatographically separated (Figure 1).
Biological activities
The authors synthesized many steroidal derivatives having 5α- reductase inhibitor activities [29,30]. It is worth to mention that in most of these derivatives the ring D of the steroid part was fused mainly with heterocyclic ring system [31,32]. The newly synthesized derivatives here belongs to a large extent to the aforementioned derivatives for their 5α-reductase inhibitors activities but these new derivatives containing pregnane nucleus where the heterocyclic fused ring system was onto ring A so it was bioassayed for their 5α-reductase inhibitors activities. So here the authors study the effects of the pregnane nucleus and fusion of heterocyclic ring system on the 5α- reductase inhibitors activities.
5α-Reductase inhibitors
All the tested compounds showed potent 5α-reductase inhibitors activities. The descending order of 5α-Reductase inhibitor activities was as follow: 3g (ED50: 0.28 μM), 3f (ED50: 0.29 μM), 3a (ED50: 0.30 μM), 3e (ED50: 0.32 μM), 3c (ED50: 0.33 μM), 3d (ED50: 0.35 μM), 3b (ED50: 0.36 μM), 3h (ED50: 0.38 μM), 4g (ED50: 0.39 μM), 4f (ED50: 0.40 μM), 4a (ED50: 0.41 μM), 4e (ED50: 0.42 μM), 4c (ED50: 0.43 μM), 4d (ED50: 0.45 μM), 4b (ED50: 0.46 μM), 4h (ED50: 0.47 μM), Anastrozole® (ED50: 1.09 μM). It was worth to mention that all tested compounds were more potent than Anastrozole® (Table 1).
| Compound No | 5α-Reductase inhibitors activities |
Anti-tumor activity against prostate cancer cell lines |
|
|---|---|---|---|
| ED50 µM | LNCaP IC50 µM | IC50 µM | |
| 3a | 0.30 | 2.56 | 9.54 |
| 3b | 0.36 | 2.90 | 10.56 |
| 3c | 0.33 | 2.77 | 9.9 |
| 3d | 0.35 | 2.87 | 10.45 |
| 3e | 0.32 | 2.76 | 9.78 |
| 3f | 0.29 | 2.45 | 7.67 |
| 3g | 0.28 | 2.34 | 7.45 |
| 3h | 0.38 | 2.91 | 10.67 |
| 4a | 0.41 | 3.10 | 11.56 |
| 4b | 0.46 | 3.56 | 13.89 |
| 4c | 0.43 | 3.39 | 12.54 |
| 4d | 0.45 | 3.47 | 12.78 |
| 4e | 0.42 | 3.29 | 11.89 |
| 4f | 0.40 | 3.01 | 11.23 |
| 4g | 0.39 | 2.95 | 10.78 |
| 4h | 0.47 | 3.76 | 14.21 |
| Anstrazol | 1.09 | 11.23 | 34.24 |
Table 1. 5α-Reductase inhibitors activities and anti-proliferate activity against prostate cancer cell lines.
Anti-proliferate activity against prostate cancer cell lines
All the tested compounds were screened as anti-tumor activities in two prostate cell lines namely, LNCaP and PC-3.
Regarding the in vitro anti prostate cancer activities against prostate cancer cell lines LNCaP the descending order of activities were: 3g (IC50: 2.34 μM), 3f (IC50: 2.45 μM), 3a (IC50: 2.56 μM), 3e (IC50: 2.76 μM), 3c (IC50: 2.77 μM), 3d (IC50: 2.87 μM), 3b (IC50: 2.90 μM), 3h (IC50: 2.91 μM), 4f (IC50: 3.01 μM), 4a (IC50: 3.10 μM), 4e (IC50: 3.29 μM), 4c (IC50: 3.39 μM), 4d (IC50: 3.47 μM), 4b (IC50: 3.56 μM), 4g (IC50: 2.95 μM), 4h (IC50: 3.76 μM), Anastrozole® (IC50: 11.23 μM). It was worth to mentioned that all tested compounds were more potent than Anastrozole® (Table 1).
Regarding the in vitro anti prostate cancer activities against prostate cancer cell lines PC3 the descending order of activities were: 3g (IC50: 7.45 μM), 3f (IC50: 7.67 μM), 3a (IC50: 9.54 μM), 3e (IC50: 9.78 μM), 3c (IC50: 9.9 μM), 3d (IC50: 10.45 μM), 3b (IC50: 10.56 μM), 3h (IC50: 10.67 μM), 4g (IC50: 10.78 μM), 4f (IC50: 11.23 μM), 4a (IC50: 11.56 μM), 4e (IC50: 11.89 μM), 4h (IC50: 14.21 μM), 4c (IC50: 12.54 μM), 4d (IC50: 12.78 μM), 4b (IC50: 13.89 μM), Anastrozole® (IC50: 34.24 μM). It was worth to mention that all tested compounds were more potent than Anastrozole® (Table 1).
Structural activity relationship
Careful examination of the relationship between chemical structure of the newly synthesized derivatives and their biological activities as 5α-reductase inhibitors, anti PC3 and anti LNCaP carcinoma activities to lead the following structural activities relationship.
1. The fusion of the pyridine heterocyclic ring system onto ring A of the steroidal scaffold is essential for 5α-reductase inhibitors, anti PC3 and anti LNCaP carcinoma activities.
2. The thiol and cyano groups provid more 5α-reductase inhibitors, anti PC3 and anti LNCaP carcinoma activities than the amino and thioamide ones due to the higher lipophilic characters of thiol and cyano groups.
3. Regarding the substitutes on the aromatic moiety the descending order of activity was 4-NO2, 2-NO2, H, 4-F, 2- Cl, 4-Cl, 4-Br, 4-OCH3.
Acknowledgement
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
References
- Arora VK, Knaus EE. Synthesis and anticonvulsant activity of 3-[3-[(dimethylamino)-methyl]-5-methyl-4H-1,2,4-triazol-4-yl]-4-(o-chlorobenzoyl)pyridine. J Heterocycl Chem 1999; 36: 201-204.
- Cesur N, Cesur Z. Synthesis of some 4-thiazoline and 4H-1,2,4-triazole derivatives of imidazo[1,2-a]pyridine as possible anticonvulsants. Farmaco 1994; 49: 679-682.
- Mosti L, Menozzi G, Schenone P, Dorigo P, Gaion R M, Belluco P. Synthesis and cardiotonic activity of 2-substituted 5-cyano-1,6-dihydro-6-oxo-3-pyridinecarboxylic acids and their methyl or ethyl esters. Farmaco 1992; 47: 427-437.
- Hojo M, Tanaka Y, Katayama O, Teramoto N. Acute antihypertensive effects of the new generation calcium antagonist 3-pyridine carboxylic acid 5-[(cyclopropylamino)-carbonyl]-1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)octyl ester on conscious spontaneously hypertensive rats and renal hypertensive rats. Arzneimforsch 1993; 43: 847-851.
- Manna F, Bolasco A, Bizzari B, Lena R, Chimenti F. Synthesis and beta-adrenoreceptor blocking activity of [[3-(alkylamine)-2-hydroxypropyl]oximino]pyridines and O[3-(alkyl- amine)-2-hydroxypropyl]methylpyridine ketone oximes derivatives. Farmaco 1996; 51: 579-588.
- Shalaby AFA, Abdalla MM, Amr AE, Hussain AA. Synthesis, reactions, and anti-arrhythmic activity of substituted heterocyclic systems using 5-chloroanisic acid as starting material. Monatshefte für Chemie 2007; 138: 1019-1027.
- Murtiashaw CW, Breitenbach R, Goldstein SW, Pezzullo SL, Quallich GJ, Sarges R. 2,3-Pyridine aphthalam. The enantioselective synthesis of an aldose reductase inhibitor. J Org Chem 1992; 57: 1930-1933.
- Amr AE, Abdel-Latif NA, Abdalla MM. Synthesis and antiandrogenic activity of some new 3-substituted androstano[17,16-c]-5`-aryl-pyrazoline and their derivatives. Bioorg Med Chem 2006; 14: 373-384.
- Abd El-Latif NA, Abdulla MM, Amr AE. Androgenic-anabolic activity of some new synthesized steroidal derivatives using 3b-hydroxy-androsten-17-one as starting material. Acta Pharmaceutica 2008; 58: 43-59.
- Amr AE, Abdulla MM. Anti-inflammatory profile of some synthesized heterocyclic aphtha and pyridine derivatives fused with steroidal structure. Bioorg Med Chem 2006; 14: 4341-4352.
- Ali HS, Al-Omar MA, Al-Khalifa AS, Abdulla MM, Ezzeldin E, Amr AE. Anti-alzheimer activity and structure activity relationship of some synthesized terpinoidal oxaliplatin analogs. J Am Sci 2011; 7: 534-542.
- Abdalla MM, Al-Omar MA, Al-Salahi RA, Amr AE, Sabry NM. A new investigation for some steroidal derivatives as anti-alzheimer agents. Int J Biol Macromol 2012; 51: 56-63.
- Brana MF, Castellano JM, Mpran M, Perez de Vega MJ, Gian XD, Romerdahl CA, Keihauer G. Bis-naphthalimides. 2. Synthesis and biological activity of 5,6-acyl-naphthalimidoalkyl-1,8-naphthalimidoalkylamines. European J Med Chem 1995; 30: 235-239.
- Amr AE, Hegab MI, Ibrahim AA, Abdalah MM. Synthesis and reactions of some fused oxazinone, pyrimidine, thiopyrimidinone and triazinone derivatives with a thiophene ring as analgesic, anticonvulsant and antiparkinsonian agents. Monatshefte für Chemie 2003; 134: 1395-1409.
- Fahmy HH, Soliman GA. Synthesis of new salicylamide derivatives with evaluation of their aphthalammatory, analgesic and antipyretic activities. Arch Pharmacal Res 2001; 24: 180-189.
- Amr AE, Mohamed AM, Ibrahim AA. Synthesis of some new chiral tricyclic and macrocyclic pyridine derivatives as antimicrobial agents. Zeitschrift für Naturforschung B 2003; 58: 861-868.
- Alagarsamy V, Meena S, Ramseshu KV, Solomon VR, Thirumurugan K, Dhanabal K, Murugan M. Synthesis, analgesic, anti-inflammatory, ulcerogenic index and antibacterial activities of novel 2-methylthio-3-substituted-5,6,7,8-tetrahydrobenzo(b)thieno[2,3-d] pyrimidin-4(3H)-ones. European J Med Chem 2006; 41: 1293-300.
- Dawood KM, Abdel-Gawad H, Rageb EA, Ellithey M, Mohamed HA. Synthesis, anticonvulsant, and anti-inflammatory evaluation of some new benzotriazole and benzofuran-based heterocycles. Bioorg Med Chem 2006; 14: 3672-3680.
- Pillai AD, Rathod PD, Xavier FP, Padh H, Sudarsanam VK, Vasu K. Tetra substituted thiophenes as anti-inflammatory agents: exploitation of analogue-based drug design. Bioorg Med Chem 2005; 13: 6685-6692.
- Starcević K, Kralj M, Piantanida I, Suman L, Pavelić K, Karminski-Zamola G. Synthesis, photochemical synthesis, DNA binding and antitumor evaluation of novel cyano- and amidino-substituted derivatives of aphtha-furans, aphtha-thiophenes, thieno-benzofurans, benzo-dithiophenes and their acyclic precursors. European J Med Chem 2006; 41: 925-939.
- Bousquet E, Romeo G, Guerrera F, Caruso A. Amico-Roxas M. Synthesis and analgesic activity of 3-substituted derivatives of pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4(3H)-ones. Farmaco Sci 1985; 40: 869-874.
- Vaille A, di Tella AM S, Maldonado J, Vanelle P. Selectivity of a CaCl2 continuous infusion screening method in rats. Methods Findings Exp Clin Pharmacol 1992; 14: 183-187.
- Marwah P, Marwah A, Lardy HA, Miyamoto H, Chang C. C19-Steroids as androgen receptor modulators: Design, discovery, and structure-activity relationship of new steroidal androgen receptor antagonists. Bioorg Med Chem 2006; 14: 5933-5947.
- Krojer M, Keller M, Bracher F. 7-Aza-des-A-steroids with antimicrobial and cytotoxic activity. Scientia Pharmaceutica 2013; 81: 329-338.
- Amaral C, Varela C, da-Silva GC, T da Silva E, Carvalho RA, Costa SCP, Cunha SC, Fernandes JO, Teixeira N, Roleira FMF. New steroidal 17β-carboxy derivatives present anti-5a-reductase activity and anti-proliferative effects in a human androgen-responsive prostate cancer cell line. Biochimie 2013; 95: 2097-2106.
- Farghaly TA, Gomha SM, Abbas EMH, Abdalla MM. Hydrazonoyl halides as precursors for new fused heterocycles of 5 α-reductase inhibitors. Archiv der Pharmazie 2012; 345: 117-122.
- George FW, Johnson L, Wilson JD. The effect of a 5 alpha-reductase inhibitor on androgen physiology in the immature male rat. Endocrinol 1989; 125: 2434-2438.
- di Salle E, Giudici D, Briatico G, Ornati G, Panzeri A. Hormonal effects of turosteride, a 5 alpha-reductase inhibitor, in the rat. J Steroid Biochem Mol Biol 1993; 46: 549-555.
- Amr AE, Abdel-Latif NA, Abdalla MM. Synthesis of some new testosterone derivatives fused with substituted pyrazoline ring as promising 5alpha-reductase inhibitors. Acta Pharmaceutica 2006; 56: 203-218.
- Al-Mohizea AM, Al-Omar MA, Abdalla MM, Amr AE. 5α-Reductase inhibitors, antiviral and anti-tumor activities of some steroidal cyanopyridinone derivatives. Int J Biol Macromol 2012; 50: 171-179.
- Abdel Hafez NA, Farghaly TA, Al-Omar MA, Abdalla MM. Synthesis of bioactive polyheterocyclic ring systems as 5α-reductase inhibitors. European J Med Chem 2010; 45: 4838-4844.
- Abdalla MM, Al-Omar MA, Bhat MA, Amr AE, Al-Mohizea AM. Steroidal pyrazolines evaluated as aromatase and quinone reductase-2-inhibitors for chemoprevention of cancer. Int J Biol Macromol 2012; 50: 1127-1132.