- Biomedical Research (2015) Volume 26, Issue 1
Prevalence of Helicobacter pylori in asymptomatic adult patients in a tertiary care hospital: A cross sectional study.
Munish Rastogi1, Dolly Rastogi2, Shraddha Singh3, Asha Agarwal4, B.P. Priyadarshi5, Tanu Middha6
1Department of Microbiology, C.S.J.M University, Uttar Pradesh, Kanpur, India
2Department of Physiology, G.S.V.M Medical College, Uttar Pradesh, Kanpur, India
3Department of Physiology, K.G Medical University, Uttar Pradesh, Lucknow, India
4Department of Pathology, G.S.V.M Medical College, Uttar Pradesh, Kanpur, India
5Department of Medicine, G.S.V.M Medical College, Uttar Pradesh, Kanpur, India
6Department of Community Medicine, G.S.V.M Medical College, Uttar Pradesh, Kanpur, India
- Corresponding Author:
- Shraddha Singh
Department of Physiology King George’s Medical University Lucknow 226003, Uttar Pradesh India.
Accepted date: June 07 2014
Abstract
Helicobacter pylori is well recognized as major cause of gastro-intestinal diseases.The present cross - sectional study was done in the department of Medicine at G.S.V.M Medical College. A total of 208 adult patients attending out patient department for symptoms other than those of gastrointestinal disorders were screened to find out the prevalence of Helicobacter pylori using Stool antigen card test. Out of these 208 patients, 92 were found positive for Helicobacter pylori by the test, giving a prevalence of 44.23%. A detailed proforma was filled, regarding the age and sex of the patient, education and occupation of the head of the family, sanitary practices, dietary habits and tobacco chewing. The patients were also examined for pallor. Among a total of 143 males, 61 were found positive for Helicobacter pylori (42.7%) and among 65 females, 31 were found positive (47.7%). Age wise distribution showed maximum prevalence of Helicobacter pylori in the age group of 30-39 years (50.7%) and minimum in the age group of more than 70 years (20%). A higher prevalence of Helicobacter pylori was found among non-vegetarians (53.3%) and among patients having pallor (52%), though both were not significant. The study of socio-economic status showed a prevalence of 42.3% in lower middle, 44.9% in upper lower and 50% in upper middle socioeconomic groups. A prevalence of 51.7% was seen among subjects chewing tobacco.
Keywords
H. pylori, stool antigen card test, pallor, tobacco.
Introduction
Helicobacter pylori, formerly known as Campylobacter pylori is a gram negative, curved, microphilic and motile organism. It colonizes and grows in human epithelial tissue and mucus. It is a common bacterium infecting about half the world’s population [1]. There is substantial evidence that it causes chronic gastritis, peptic ulcers, and duodenal ulcers and is also involved in the development of gastric carcinoma [2-4]. Helicobacter pylori was identified in 1984 [5] and further it was classified as carcinogenic to humans by The International Agency for Research on Cancers in next 10years [6]. Actual infection rates vary from nation to nation and the developing world having higher rates than the developed countries [1,2]. The age at which this bacterium is acquired seems to influence the possible pathologic outcome of the infection.
Infections are usually acquired in early childhood in most of the countries [7]. Acquisition at an older age brings different gastric changes more likely to lead to duodenal ulcers [8]. In industrialized countries almost 50% of adults are infected, although its prevalence seems to be decreasing [9]. However, in developing countries the prevalence is higher and as much as up to 90% figure has been reported [10,11]. Once acquired, Helicobacter pylori infection generally persists throughout life, unless treated by specific antimicrobial therapy [10]. Helicobacter pylori consist of a large diversity of strains and genomes [12-16].The study of genome is focused to understand the ability of this organism to cause disease. Twenty nine percent of the loci are in the ‘Pathogenic’ category of genome database and two of the sequenced strains have an approximately 40 Kb long cytotoxin associated gene pathogenicity island (cag PAI), which a common gene sequence responsible or pathogenesis is containing over 40 genes. This cag PAI is usually absent from Helicobacter pylori strains isolated from humans who are carriers of Helicobacter pylori but remain asymptomatic [17]. The cytotoxin associated gene ( cagA )gene codes for one of the major Helicobacter pylori virulence proteins. Bacterial strains that have the cytotoxin associated gene ‘CagA’ gene are associated with an ability to cause ulcers [18]. It is important to state that many individuals who might be harboring the bacterium do not develop any clinically apparent disease.
Several modes of transmission of Helicobacter pylori are suspected and no single pathway has been clearly identified. It has been demonstrated that housefly has the potential to transmit Helicobacter pylori mechanically [19] and thus poor sanitation may potentiate its spread. Person to person contact is considered to be the most likely transmission route. Another important mode of transmission is iatrogenic in which tubes or endoscopes that have been in contact with gastric mucosa of one individual are used for another patient [20]. Occupationally acquired infections have also been reported especially among, endoscopists and gastroenterologists [10,20-22]. Another possible route is orofecal and Helicobacter pylori have been isolated from faeces of infected young children [10,21].
There are also studies investigating the association between the seroprevalence of Helicobacter pylori and Hepatitis A virus [23-28]. Water contaminated with faeces may be a major source of infection as is consumption of uncooked vegetables irrigated with water contaminated with seropositivity [29]. Contaminated municipal water supply has greater chances of spreading Helicobacter pylori infection as compared to private water supply [30]. Sporadic isolates have been found from dental plaques and saliva [31,32]. Various socio-economic conditions comprising of high density crowding, poor sanitary practices, family income, educational level and occupation [33-35] have been held responsible in spreading of the pathogen. In developing countries, factors related to community and religion might also be responsible [36]. Early detection of Helicobacter pylori population and its eradication may result in significant improvement in severity of dyspeptic symptoms. It is important to find out Helicobacter pylori prevalence and identify high-risk population so that treatment strategies can be appropriately planned. This becomes even more important for those patients who are harbouring Helicobacter pylori but are asymptomatic.
Hence the present hospital-based cross-sectional study was done on patients attending OPD for conditions other than gastro-intestinal disorders and the prevalence of Helicobacter pylori was estimated. Other parameters like socio-economic status according to Kuppuswamy grading, age, sex, diet, pallor and tobacco consumption were also studied and association of Helicobacter pylori if any, was identified.
Materials and Methods
The study was conducted in the department of Medicine at GSVM Medical College, Kanpur in collaboration with de partment of Physiology at KGMU, Lucknow during 2012- 2013. A total of 208 patients of both sexes attending OPD for symptoms other than those of gastrointestinal disorders were screened for Helicobacter pylori .Written informed consent was taken from all the patients after explaining to them the nature and purpose of study. Ethical clearance was taken prior to the study from the ethical committee. Patients who had taken proton pump inhibitors or antibiotic for a month prior to study were excluded. Patients’ stool samples were collected in airtight containers and stool assay was performed using Immunocard STAT HpSA test (Standard diagnostics Inc).
The test device and the sample were brought to room temperature prior to testing. The test device was laid on a flat dry surface and about three drops of the prepared sample were poured into the sample well. Interpretation was done after fifteen minutes. Two colour bands, one at test band and another at control band indicated a positive result. Negative test results showed only control band. HpSA test is a non-invasive, accurate test especially useful for screening of asymptomatic subjects. Pallor was seen in the lower palpebral conjunctiva.
Statistical Analysis
Data were analysed by the chi-square test to compare the association between different variables and positive Helicobacter pylori rates. A value of P <0.05 was considered statistically significant. Calculations were done using the software package SPSS 16.0.
Results
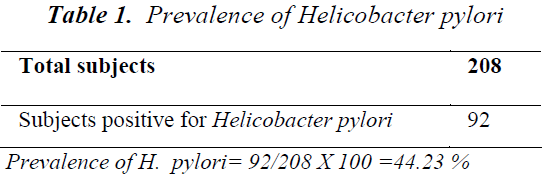
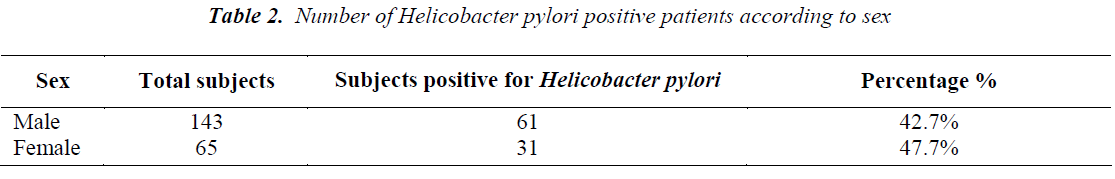
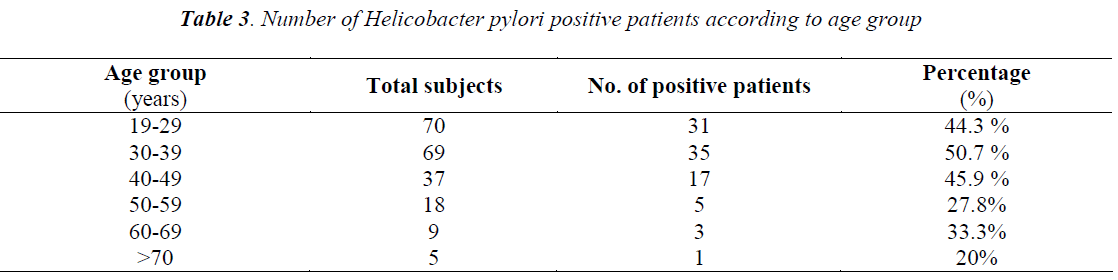
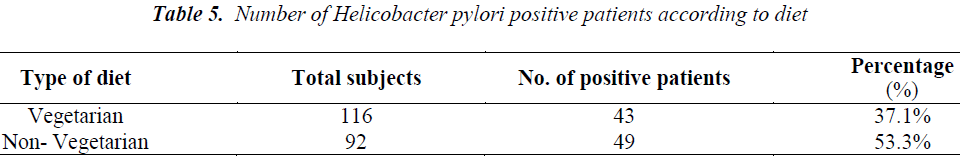
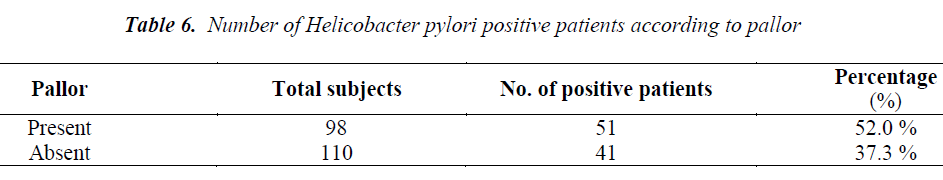
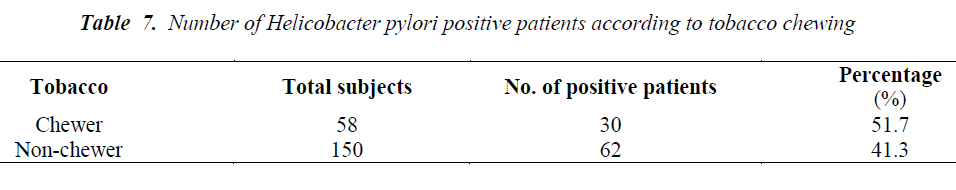
Out of total 208 patients, 92 patients were Helicobacter pylori positive by Immunocard STAT HpSA test, giving a hospital based prevalence of 44.23% (Table 1). Out of total 143 males, 61 were positive for Helicobacter pylori (42.7%) whereas out of 65 females 31 were positive (47.7%) (Table 2). The prevalence was estimated in different age groups. The maximum number of positive patients was found in the age group of 30-39 years (50.7%) and the minimum prevalence was in the age group of above 70 years (20%) (Table 3). For socio economic status, the groups were classified according to modified Kuppuswamy scale for urban families. Out of 118 patients belonging to upper lower socioeconomic group, 53 were positive for Helicobacter pylori (44.9%), out of 78 of lower middle group, 33 were positive (42.3%) and out of 12 of upper middle vegetarians with 43 positive (37.1%) (Table 5). Pallor was present in 98 patients with 51 positive (52.0 %) and absent in 110 patients with 41 positive for H.pylori (37.3%) (Table 6). Out of 58 tobacco-chewing subjects, 30 were positive for Helicobacter pylori, which indicated a prevalence of 51.7%. (Table 7).
Discussion
The prevalence of Helicobacter pylori infection varies worldwide, but higher colonization rates have been seen in developing countries, compared to developed countries. This study was conducted to find out the prevalence of H.pylori among patients attending OPD for symptoms other than gastrointestinal disorders. These patients were screened for Helicobacter pylori by Immunocard STAT HpSA test. In a study from Chandigarh, 254 individuals were screened for Helicobacter pylori which was positive in 56.7% asymptomatic individuals [37]. The overall prevalence recorded in our study was 44.23%, which is less in comparison to the above study. This can be explained by the fact that prevalence of H.pylori varies widely by geographic area, age, race, and ethnicity and SE status. In another study, Helicobacter pylori prevalence was seen in patients with dyspepsia and in control subjects to be 65% and 46% respectively [38]. In a study conducted on healthy Omani blood donors, the overall prevalence for Helicobacter pylori was 69.5% [39]. Similar results were shown in a study from Turkey where 53% asymptomatic subjects were seropositive for Helicobacter pylori antibodies [40], whereas 51% asymptomatic subjects were seropositive in a study from Saudi Arabia [41]. In the present study, among Helicobacter pylori positive patients 42.7% were males and 47.7% were females. Although there is a slightly greater female preponderance but the difference between the genders was not significant which goes in accordance with a similar study from South India [42] and from other parts of the world [40,43,44]. However, in a study from Oman [39], Helicobacter pylori seropositivity has shown increasing tendency with age in females (55- 65%) as compared to males of the same age group (35- 55%). In another study, attention was given to gender differences indicating that prevalence of Helicobacter pylori infection was higher in men with upper and non-upper digestive tract symptoms than that in women [45]. In our study, age wise distribution showed maximum prevalence in the age group of 30-39 years (50.7%) and minimum in the age group of more than 70 years (20%). This goes in accordance with a similar Indian study in which the maximum prevalence was in the age group of 36-45 years (43.47%) and minimum in the age group of 66-75 years (3.26%) [46]. An early study from Saudi Arabia found an increase in seroprevalence of Helicobacter pylori with advancing age reaching to 70% for those who were 20 years old or more [47].An increase in prevalence with age being maximum (74%) between 16-30 years and thereafter showing a slight decline has been reported in another Indian study[42].In a study from Mumbai, age related prevalence of Helicobacter pylori showed similar results as ours with maximum prevalence of 58% in the age group of 30- 39 years [38].
In the present study, out of 98 patients having pallor 51 patients were positive for H. pylori (55.4%) (p>0.05). Helicobacter pylori colonization appears to impair iron uptake and increase iron loss. Regarding the possible role of Helicobacter pylori in iron deficiency anaemia, a recent metaanalysis indicated that the infection is associated with depleted iron deposits. The mechanism by which Helicobacter pylori induces this alteration is not clear but it appears to involve GI blood loss, diminished iron absorption from diet and increased consumption of iron by the bacteria [48]. A study with Helicobacter pylori infected patients from Bangladesh has shown the prevalence of iron deficiency anaemia with a decrease in haemoglobin while serum ferritin was significantly higher in [49]. The prevalence of Helicobacter pylori in our study was found to be higher in low socioeconomic group being 57.6% in upper lower and 35.9% in lower middle groups. This is consistent with previous studies which have demonstrated that the prevalence of Helicobacter pylori as well as gastritis is more frequent in those who come from large families, have poor hygiene, low standards of living, poor sanitation practices and over crowded living conditions [50,51,52]. Socioeconomic status is not restricted to income and social class but also considers other factors such as living standards, urbanization and educational level [53]. Similar result was seen in a study among professional workers of Kashmir valley [54]. A prevalence of 46.7% was seen in vegetarians and 53.3% in non-vegetarian group, which was though higher in non-vegetarians but was not significant (p>0.05). This supports the fact that it is probably the food prepared under unhygienic conditions that plays a role in transmission of Helicobacter pylori in developing countries and not the type of food consumed [55]. Similarly, the prevalence of Helicobacter pylori among tobacco chewers was higher (51.7%) than among non-tobacco chewers (41.3%) but was not significant (p>0.05). Both non vegetarian diet and tobacco chewing have been studied as risk factors for peptic ulcer and gastric cancer among Helicobacter pylori infected individuals [56-58] but still more studies are required to establish a association between them.
Conclusion
The present study revealed substantial prevalence of Helicobacter pylori in asymptomatic patients with females being more affected than males and a maximum prevalence in the age group of 30-39 years. The prevalence is higher in low socioeconomic classes with poor sanitation practices and unhygienic water supply. A higher prevalence of Helicobacter pylori seen in subjects having pallor may be contributed to poor iron absorption. Similarly, a higher prevalence of Helicobacter pylori was noticed among non-vegetarians and tobacco consumers, which may be the contributing factors in the development of peptic ulcer and gastric cancer in patients harboring Helicobacter pylori. Identification of populations, who do not show symptoms of Helicobacter pylori infection, but still harbour it, is essential for controlling the infection and it still remains a challenge for the clinicians.
Acknowledgement
We are sincerely thankful to the faculty of KGMU, Lucknow and GSVM Medical College, Kanpur for their valuable support and guidance. I also extend my gratitude to Standard Diagnostics Inc for providing me the kits for Helicobacter pylori.
References
- Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries" Aliment Pharmacol Thera 1995; Volume? (Suppl 2): 33-39.
- Labigne A; de Reuse H. Determinants of Helicobacter pylori pathogenecity. Infectious Agents and Diseases 1996; 5: 191-202.
- McColl KEL. Helicobacter pylori, clinical aspects. Journal of Infection 1997; 34: 7-13.
- Riegg SJ, Dunn BE, Blaser MJ. Microbiology and pathogenesis of Helicobacter pylori.. Infections of the gastrointestinal tract. New York. Raven Press. 1995; 535-550.
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and ulceration. Lancet 1984; i: 1311-1315.
- International- Agency for Research on Cancers. Monographs on the evaluation of carcinogenic risks to humans. Geneva World Health Organisation. 1994; Vol. 61.
- Kusters JG, Van Vliet AH, Kuipers EJ Pathogenesis of Helicobacter pylori infection". Clinical Microbiol Rev July 2006 19(3): 449-490.
- Brown LM. Helicobacter pylori epidemiology and routes of transmission" Epidemiol Rev 2000 22(2): 283-297.
- Farthing NJG. Helicobacter pylori infection : an overview. British Medical Bulletin. 1998, 54: 1-6.
- Dunn BE, Cohen H, Blaser MJ, Helicobater pylori Clinical Microbiology Reviews. 1997, 10: 720-741.
- Bardhan PK: Epidemiological features of Helicobactor pylori infection in developing countries. Clinical Infectious Disease. 1997, 25: 973-978.
- Tomb JF, White 0, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD. The complete genome sequence of the gastric pathogen Helicobactor pylori" August 1997: Nature 388 (6642): 539-547.
- "Genome information for the H. pylori 26695 and J99 Strains Institute Pasteur 2002 Retrieved 2008-09-01.
- H. pylori 26695 complete genome" National Center for Biotechnology information, Retrieved 2008-09-1.
- "H pylori J 99 Complete genome" National Center for Biotechnology information Retrieved 2008-09-01.
- "Oh JO, Kling- Backhed H, Giannakis M, Xu J, Fulton RS, Fulton LA, Cordum HS, Wanq C, Elliott G, Edwards J, Mardis ER, Enqstrand LG, Gordon JI. The complete genome sequence of a chronic atrophic gastritis H. pylori strain: Evolution during disease progression". Proc Nat. Acad Sci U.S.A. June 2006: 103(26): 9999-10004.
- Baldwin DN, Shepherd B, Kraemer P, Hall MK, Sycuro LK, Pinto-Santini DM, Salama NR. Identification of H. pylori genes that contribute to stomach." Colonization II infec Immun Feb 2007 75(2): 1005-1016.
- Broutet N, Marais A, Larnouliatte H Mascarel A, Samoyean R, Salamon R, Megraud F)" Cag A status and Eradication Treatment. Outcome of Anti H. pylori Triple Therapies in patients with nonulcer dyspepsia. J Clin Microbiol April.2001: 39(4): 1319 – 22.
- Grubel P Hoffman JS, Chong FK, Burstein NA Mepam C, Cave DR. Vector Potential of houseflies (Musca domestica) for Helicobacterpylori. Journal of Clinical Microbiology. 1997, 35: 1300-1303.
- Akamatsu T, Tabata K, Hironga M ,Kawakami H, Yyeda M. Transmission of Helicobacter pylori infection via flexible fiberoptic endoscopy, American Journal of infection control. 1996, 24: 396-401.
- Lin SK, Lambert JR, Schembri MA ,Nicholson L, Korman MG. Helicobacter pylori prevalence in endoscopy and medical staff. Journal of Gastroenterology and Hepatology. 1994, 9: 319-324.
- Chong J, Marshall BJ, Barkin JS ,McCallum RW ,Reiner DK., Hoffman SR, O Phelan C. Occupational exposure to H. pylori for the endoscopy professional: a sera epidemiological study. American Journal of Gastroenterology. 1994, 89: 1987-1992.
- Rudi J, Toppe H, Marx N, Zuna I, Theilmann L, Stremmel W, Raedsch R. Risk of infection with helicobacter pylori and hepatitis and virus in different groups of hospital workers. American journal of Gastroenterology. 1997, 92: 258-262.
- Sathar MA, Gowws E, Simjee AE ,Mayat AM.Seroepidemiological study of helicobacter pylori infection in south African children transaction of the royal college of medicine and hygiene; 1997; 42: 258-262.
- Handt LK, Fox JG, Dewhirst FE, Fraser GJ, Paster BJ, Yan LL.:Helicobacter pylori isolated from the domestic cat:publc health implications infection and immunity. 1994; 612; 2367-2374.
- Fox JG .Non-human reservoir of helicobacter pylori. Alimentary Pharmacology and Therapeutics. 1995; 9 (suppl.2): 93-103.
- Webb PM, Knight T, Elder JB, Newell DG, Forman D. Is Helicobacter pylori transmitted from cats to humans? Helicobacter. 1996; 1: 79-81.
- Furuta T, Kamata T, Takashima M, Futami H, Arai H, Hanai H. .Study of transmission routes of H.pylori in relation to seroprevalence of hepatitis and virus. Journal of clinical Microbiology. 1997, 35: 1891-1893.
- Hopkins RJ, Vial PA, Ferreccio C, Ovalle J, Prado P, Sotomayor V, Rusell. Seroprevalence of helicobacter pylori in chile: Vegetables may serve as one route of transmission Journal of infectious Disease 1993. 168: 222-226.
- Klein PD, Opekun AR, Smith EO, Graham DY, Gaillour A. Water source as risk factor for helicobacter pylori infection in Peruvian children Gastrointestinal Physiology working group. Lancet 1991, 337: 1503- 1506.
- Namavar F, Roosendaal R, Kuipers EJ. Presence of helicobacter pylori in the oral cavity, oesophagus. Stomach and faeces of patients with gastritis. European Journal of clinical Microbiology and infectious Disease, 1995, 14: 234-237.
- Megraud F. Transmission of helicobacter pylori. Foecal oral route Alimentary Pharmacology and Therapeutics. 1995, 9(suppl-2): 85-91
- Peach HG, Pearce DC, Farish SJ Helicobacter pylori infection in an Australia regional city: Prevalence and risk factor .Medical Journal of Australia. 1997, 167: 310-313.
- Malaty HM, Paykov V, Bykova O, Ross A, Graham DP, Anneger JF, Graham DY. Helicobacter pylori & socio-economic factors in Russia, Helicobacter. 1996, 1: 82-87.
- Rothenbacher D, Bode G, Winz T, Berg G, Adler G, Brenner H. H. pylori in out patients of a general, practitioner. Prevalence and determinants of current infection. Epidemiology and infection.1997, 119: 151-157.
- Lindkvist P, Enquselassie F, Asrat D, Muhe L, Nilsson I, Giesecke J. Risk factor for infection with H. pylori-a study of children in rural Ethiopia Scandinavian Journal of Infectious Disease.1998, 30: 371-376.
- Singh V, Trikha B, Nain C.K. Epidemiology of Helicobacter pylori & peptic ulcer in India. J Gastroenterol Hospital 2002; 17 (6): 659-665.
- Gill HH, Desai HG, Majumdar P, Mehta PR, Prabhu SR. Epidemiology of Helicobacter pylori: the Indian Scenario. Ind j Gastroenterol 1993 Jan; 12(1): 9-11.
- Mohammed Said Al-Balushi, Juma Zal-Busaidi, Muna S Al-Daihani, Mohammed O, Shafeeq, Sidgi S. Hasson. Sero prevalence of Helicobacter pylori infection among asymptomatic healthy Omani blood donors. Asian Pacific Journal of Tropical Disease 2013; 3(2): 146-149.
- Us D, Hascelik G. Seroprevalence Helicobacter pylori infection in asymptomatic Turkish population. J Infect 1998; 37: 148-150.
- Mubashir A Khan, Hani O. Ghazi. Helicobacter pylori infection in asymptomatic subjects in Makkah, Saudi Arabia JPMA57: 2007; 114-117.
- V Kate, N Ananthakrishnan, C Ratnakar, S Badrinath. Anti - H Pylori IgG Seroprevalence rates in asymptomatic children & adults from South India. Indian J Med Microbiol 2001; 19: 20-25.
- Soheila Montazer-Saheb, Safar Farajnia, Nazli Saeedi, Rana Yousefzadeh, Abbas Rafat, Leila Rahbarnaei. Seroprevalence of Helicobacter pylori infection in patient suffering from gastric symptoms in North West of Iran. African Journal of Microbiology research, Oct. 2011; 5(22): 3616-3619.
- Bakka AS, Salih BA. Prevalence of Helicobacter pylori infection in asymptomatic subjects in Libiya Diagn. Microbiol Inf Dis 2002; 43: 265-268.
- Broutet N, Sarasqueta AM, Sakarovitch C. Helicobacter pylori infection patients consulting gastroenterologists in France: Prevalence is linked to gender and region of residence. Eur J Gasteroenterol Hepatol 2001; 13: 677-684.
- Kumar R, Bano G, Kapoor B, Sharma S, Gupta Y. Clinical profile in Helicobacter pylori positive patients in Jammu. J.K. Science; 2006; 8(3): 148-150.
- Al-Mogel MA, Evans DG, Evans DJ, Abdulghani ME. Prevalence of Helicobacter pylori infection in Saudi Arabia & comparison of those with and without upper gastrointestinal symptoms. Am J Gastroenterol 1990; 85: 944-948.
- Fernando Bermejo, Santiago Garcia - Lopez. A guide to diagnosis of iron deficiency and IDA in digestive diseases. World J Gastroenterol 2009. October 7; 15 (37): 4638-4643.
- Shamima Sultana, Shafiqul A. Saker Samina Sattar. Serum ferritin, Hemoglobin, Soluble Transferrin Receptor and Helicobacter pylori infection in periurban community children in Bangladesh. 8th CCDM www.icddrb.org.Scientific Session 5 - Helicobacter pylori 024 (109).
- David Y. Graham, Hoda M. Malaty Dolores G. Evans. Epidemiology of Helicobacter pylori infection in asymptomatic population in the United States. Effect of age race, and socioeconomic status. Gastroenterology 1991; 100: 1495-1501.
- Edwards FC, Goghill NF. Aetiological factors in chronic atrophic gastritis - Br Med J 1966; 2: 1409- 1415.
- Massarrat S, Paidlik A, Pittner P, Schmitz - Moorman P, Wurbs M. The role of certain habits and various diseases in the occurrence of gastritis. Hepatogastroenterology 1983; 30(6): 249-253.
- Khalifa MM, Sharaf RR, KK Aziz. Helicobacter pylori: a poor man's gut pathogen? Gut pathogens 2010, 2: 2.
- Parray S., Malik S. Basu J, Rashid S. Malik G.M. Helicobacter pylori prevalence in professional workers in main teaching hospitals of Kashmir valley. J.K. Practitioner April-June 2006; 13(2): 95-97.
- Rodolfo E. Begue, Jose L. Gonzales Herman Correa - Gracian, Si. Chin Tang. Dietary risk factors associated in the transmission of Helicobacter pylori in Lima, Peru. AM J. Trop. Med. Hyg. 1998; 59(4): 637-640.v
- Siman JH, Forsgren A, Berqland G. Floren CH. Tobacco smoking increase risk for gastric adenocarcinoma among Helicobacter pylori infected individuals. Scand J Gastroenterol 2001 Feb; 36(2): 208-213.
- Ishita L, Pankaja SS, Sandeep M, Vikram K. A study of Helicobacter pylori infection, dietary pattern and habits in patients with gastric cancer in South India. Asian pacific Journal of Tropical disease 2012; 24-26.
- Hong ZD, Liya H, Sanren L, Ganj DS. Recent changes in the prevalence of Helicobacter pylori infection among children and adults in high or incidence region of gastric cancer in China. Chinese Medical Journal 2009; 122(15): 1759-1763.