- Biomedical Research (2010) Volume 21, Issue 2
Cortisol: Testosterone ratio and homocysteine levels in patients with acute myocardial infarction
Shahid A. Mujawar1*, Vinayak W. Patil1, Rekha G. Daver2
1Department of Biochemistry, Mumbai, Maharashtra, India.
2Department of obstetrics and Gynecology, Grant Medical College and Sir J.J. Group of Govt. Hospitals, Byculla, Mumbai, Maharashtra, India.
- *Corresponding Author:
- Shahid A. Mujawar
Flat No-30, 7th Floor, Swstik-4
Sir J.J. Hospital Campus
Byculla, Mumbai 400008.
Tel: +91-9987793277
E-mail: akbarson4@gmail.com
Abstract
Stressful experiences include major life events, trauma, and abuse and are sometimes re-lated to the environment in the home, workplace, or neighborhood. Acute stress and chronic can both have long-term consequences. Therefore, this study was carried out to investigate cortisol, total testosterone and total homocysteine (tHcy) status in acute myocardial infarc-tion (AMI). Thirty AMI patients were included and diagnosed based on clinical examina-tion, laboratory investigations, electrocardiogram, angiography and other tests. The blood samples were analyzed for cortisol, total testosterone and tHcy. Significantly increased levels of serum cortisol and tHcy, and decreased level of total testosterone were noticed in the pa-tients group as compared to control subjects. Whereas, we found positive and significant correlation between cortisol : testosterone (C/T) ratio and tHcy, and mean blood pressure in AMI patients. This study suggests that, some methods require which reduces the C/T ratio and homocysteine levels may reduce the risk of AMI.Key Words
Acute myocardial infarction, cortisol : testosterone ratio, homocysteine
Introduction
In Asian countries, cardiovascular diseases (CVD) are a major public health problem. Despite the increase in CVD mortality rate during the recent decade, over 40% of the death are still caused by CVD in India [1, 2] Obesity, hypercholesterolemia, smoking and hypertension have been recognized as major risk factors for cardiovascular diseases; however, they do not fully explain the pathogenesis and causality of these diseases. A new class of emerging risk factor for cardiovascular diseases is homocysteine plasma levels [3].
Neuroendocrine mechanisms are involved in the production of social inequalities in coronary heart disease. Reduced variability in heart rate, indicating predominance of sympathetic over parasympathetic activity, is linked with adverse work characteristics and anxiety and separately with increased risk of sudden death [4]. Depression, which is linked with excessive production of glucocorticoids, predicts future coronary disease [5]. Cushing's syndrome is characterized by central obesity and increased risks for hypertension, diabetes, and coronary disease. Central obesity is linked with both of these degenerative diseases and is a feature of low socioeconomic status. The metabolic syndrome of central obesity, glucose intolerance, insulin resistance, lipoprotein disturbances, and reduced fibrinolysis, secondary to altered functioning of the hypothalamic-pituitary-adrenocortical axis, may mediate effects of the psychosocial environment on coronary risk [6]. The challenge of a car driving simulation showed that, consistent with a neuroendocrine conditioning effect, low reactivity was linked with high self esteem. Assessed by a standard psychometric method, self esteem was unre-lated to cortisol concentration at baseline but was inversely related to the amount of increase during the challenge [7].
The contribution of stress to coronary heart disease (CHD) risk has been investigated for many years, but considerable disagreement remains about whether stress influences CHD and, if so, the relative importance of this compared with other CHD risk factors [8,9]. Our aim was to assess the changes in serum concentrations of cortisol, testosterone and homocysteine levels after AMI and to evaluate the diagnostic utility of these parameters as early non-invasive markers of stress.
Material and Methods
This study was carried out at Department of Biochemistry, Grant Medical College and Sir J. J. Group of Government Hospitals, Mumbai. All participants completed a medical history form and provided informed consent. The presence of coronary heart disease at baseline was determined by the Rose questionnaire for myocardial infarction, the electrocardiogram results, and a self-reported confirmation of heart disease diagnosed by a physician [10]. Thirty (30) AMI patients were included in the present study. All the AMI patients were diagnosed based on clinical examination, laboratory investigations, electrocardiogram, angiography and other tests. The control group consist of 30 normal healthy subjects, having age ranging 20 to 65 years matched with the patients. All the controls were symptom less on clinical examination.
Inclusion criteria were normal renal and liver function. Exclusion criteria were use of medications (therapy involving Prednisolone, Corticosteroid, S-adenosylmethionine, carbamazepine, phenytoin, 6-azauridine, anthopterin, antifolates, anticonvulsant agents, tamoxifen, and theophylline), diabetes mellitus, cancer, anemia, and systemic illness. The Institutional Ethical Committee at the Grant Medical College and Sir J.J. Group of Government Hospitals, Mumbai, India, approved the study.
Blood sample collection
Venous blood samples were collected in test tube with aseptic precautions. After 2 hours of collections sample was centrifuged at 3000 rpm for 5 minutes. Serum was separated and collected in polythene tube with cork. The sera with no sign of hemolysis used for the analysis of cortisol, total testosterone and total circulating homocysteine (tHcy).
Biochemical Analysis
Serum cortisol estimated by the method of solid-phase, competitive chemiluminescent enzyme immunoassay. [11]. Serum total testosterone concentration was evaluated by solid-phase, competitive chemiluminescent enzyme immunoassay method [12]. Serum tHcy concentration was measured by competitive chemiluminescent enzyme immunoassay method [13]. We used fully automated enzyme amplified chemiluminescent immunoassay based Immulite 1000 analyzer. Measurement of these blood parameters by using commercial kits from Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA.
Statistical Analysis
Numerical variables were reported in terms of mean and standard deviation. Statistical analysis of results was done by normal ‘z’ test. In this analysis, variables showing p value less than 0.05 and 0.001 were considered to be statistically significant and highly significant respectively. Pearson correlation test was used to test.
Results
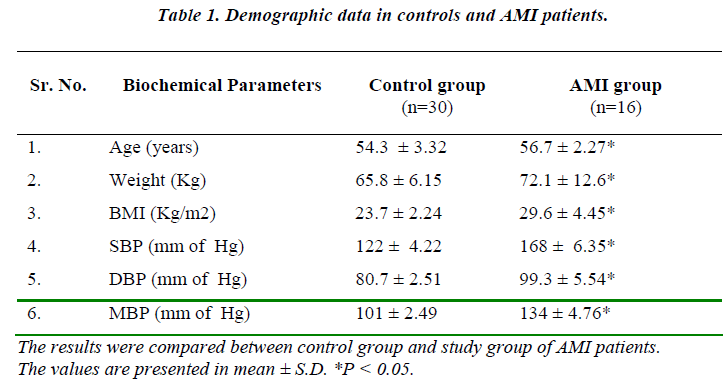
Demographic data of AMI patients such as age, weight, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean blood pressure (MBP) are significantly change (p< 0.05) as compared with normal control group (Table 1).
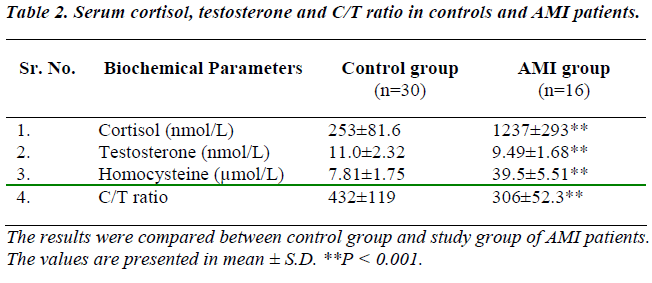
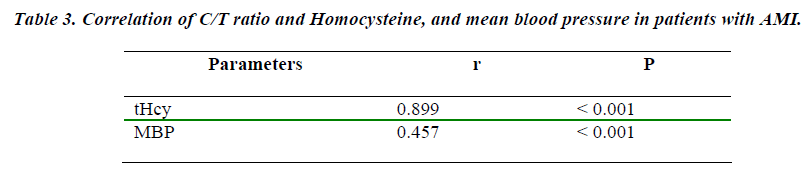
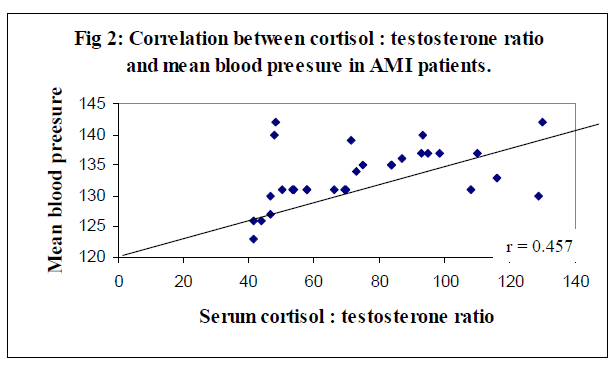
Table 2 shows levels of serum cortisol, testosterone, homocysteine, and C/T ratio in healthy subjects and AMI patients. Study group showed a significant (p<0.001) increase in serum cortisol, homocysteine, and C/T ratio whereas, significant (p<0.001) decrease in serum testosterone (p<0.001 as compared to control subjects. Positive and significant (p < 0.001) correlations were observed between levels of cortisol : testosterone ratio and homocysteine, and MBP. (Table 3).
Discussion
Excessive cardiovascular reactivity to stress may have a pathophysiological role in neurogenic hypertension [14]. In study group, age, weight, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean blood pressure (MBP) were showed significant (p < 0.05) increase. The present study shows that there was significant change in adrenal and cardiac functions in patients with AMI.
Serum cortisol level was significantly increased (p<0.001) in cases of AMI. Kumari et al [8] studied animal model of stress-induced acceleration of atherosclerosis that provides scope for future studies into its etiology/pathogenesis, the mechanisms through which stress can increase disease risk are currently poorly understood.
Cortisol is the potential mediator between stress and cardiovascular disease that has been most discussed in the literature. Four small cross sectional studies suggested that early-morning plasma cortisol levels correlate with the degree of coronary artery disease detected on angiograms [15-17], but studies failed to find this association [18]. There is limited additional evidence from studies comparing poorly characterized groups of patients with various diseases (including cardiovascular disease) with subjects without these diseases, which suggests that blood cortisol levels may be related to cardiovascular disease [19]. Endogenous corticosteroid treatment has also been associated with elevated cardiovascular disease risk [20], and evidence from animal studies suggests detrimental effects on cardiovascular health of elevated cortisol [21].
Serum testosterone concentration was significantly (p<0.001) decreased in AMI patients whereas cortisol : testosterone ratio were significantly (p<0.001) increased in our study group as compared with normal subjects. Cortisol and testosterone secretion are interrelated. Activation of the hypothalamo-pituitary-adrenal axis not only results in an elevation of adrenal corticosteroids but also inhibits gonadotrophin secretion [22], which will result in a secondary reduction of testosterone concentration in the male. Therefore, decreased testosterone and increased C / T ratio in AMI which corroborates with the other studies, [23-27] is a useful proximal marker of processes that may lead to increased risk of CVD.
Hyperhomocysteinemia, in such AMI patients was found in our study might to be due to alteration of cardiac func-tions. Homocysteine is a sulphur-containing amino acid that results as an intermediary product of the methionine degradation pathway. The folic acid and vitamin B12 or B6 deficiencies can play a role as cofactors that increase homocysteine levels. Additional studies have shown a negative correlation between homocysteine levels and folic acid and vitamin B12 and B6 plasma levels [28,29]. In this study we cannot estimate serum vitamin B. Several studies have been performed to demonstrate association between homocysteine levels and cardiovascular diseases which corroborates with the work of Gravina-Taddei, Yadav et al [3,31].
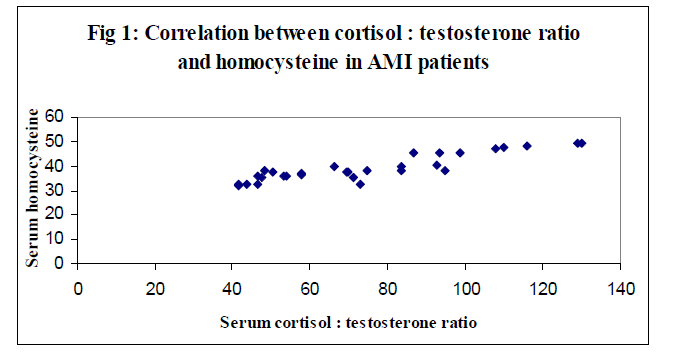
Better correlation between the cortisol : testosterone ratio and homocysteine in overall AMI patients was statistically positive (r = 0.899) and significant (p < 0.001) were observed (Figure 1). The correlation coefficient (r) 0f 0.457 (p > 0.001) between the serum cortisol : testosterone ratio and MBP level was good (Figure 2). These correlations were in agreement with the findings of Smith et al [31].
We can conclude that biochemical screening such as cortisol, testosterone and homocysteine is of paramount importance in all AMI patients. This study suggests that, some methods require which reduces the C/T ratio and homocysteine levels may reduce the risk of AMI. On the other hand, there is an absolute need for large studies designed to answer the question as to whether adrenal dysfunction are associated with increased risk for CVD and whether therapy of these disorders might influence cardiovascular mortality. Further studies should help define the role of genetic polymorphism in increased cortisol, reduced testosterone and homocysteine metabolism enzymes and their role in AMI patents.
References
- World Health Organization: Reducing Risks, Promoting Healthy Life. World Health Report 2002. World Health Organization: Geneva; 2002.
- Gupta PC, Ball K. India: Tobacco Tragedy. Lancet 1990; 335-595.
- Gravina-Taddei CF, Batlouni M, Sarteschi C, Baltar VT, Salvarini NAC, Bertolami MC, et al. Hyperhomocys-teinemia as a risk factor for coronary atherosclerotic diseases in the elderly. Arq Bras Cardiol 2005; 85: 166-173.
- Algra A, Tijssen JGP, Roelandt JRTC, Pool J, Lubsen J. Heart rate variability from 24-hour electrocardiography and the 2-year risk for sudden death. Circulation 1993; 88: 180-185.
- Anda R, Williamson D, Jones D, Macera C, Eaker ED, Glassman A, et al. Depressed affect, hopelessness, and the risk of ischaemic heart disease in a cohort of US adults. Epidemiol 1993; 4: 285-294.
- Bjorntorp P. Visceral fat accumulation: the missing link between psychosocial factors and cardiovascular dis-ease? J Int Med 1991; 230: 195-201.
- Seeman TE, Berkman LF, Gulanski BI, Robbins RJ, Greenspan SL, Charpentier PA, et al. Self-esteem and neuroendocrine response to challenge: MacArthur stud-ies of successful aging. J Psychosom Res 1995; 39: 69-84.
- Kumari M, Grahame-Clarke C, Shanks N, Marmot M, Lightman SL, Vallance P Chronic stress accelerates atherosclerosis in the apolipoprotein E deficient mouse. Stress 2003; 6: 297-299.
- MacLeod J, Davey Smith G, Heslop P, Metcalfe C, Car-roll D, Hart C. Are the effects of psychosocial exposures attributable to confounding? Evidence from a prospec-tive observational study on psychological stress and mortality. J Epidemiol Community Health 2001; 55: 878-884.
- 10. Welborn TA, Cumpston GN, Cullen KJ, Curnow DH, McCall MG, Stenhouse NS. The prevalence of coronary heart disease and associated factors in an Aus-tralian rural community. Am J Epidemiol 1969; 89: 521-536.
- Foster L, Dunn R. Single antiboby technique for radio-immunoassay of cortisol in unextracted serum or plasma. Clin Chem 1974; 20: 365-368.
- Jaffe BM, Behrman NR, editors. Methods of hormone radioimmunoassay. Academic Press, 1974.
- Ueland PM, Refsum H, Stabler SP, et al. Total homo-cysteine in plasma or serum: Methods and clinical ap-plications. Clin Chem 1993; 39: 1764-1779.
- Matthews KA, Weiss TM, Detre T, Dembroski TM, Falkner B, Manuck SB, Williams RB, eds. Handbook of Stress, Reactivity, and Cardiovascular Disease. New York, NY: John Wiley & Sons Inc; 1986.
- Troxler RG, Sprague EA, Albanese RA, Fuchs R, Thompson AJ. The association of elevated plasma corti-sol and early atherosclerosis as demonstrated by coro-nary angiography. Atherosclerosis 1977; 26: 151-162.
- Koertge J, Al-Khalili F, Ahnve S, Janszky I, Svane B, Schenck- Gustafsson K. Cortisol and vital exhaustion in relation to significant coronary artery stenosis in middle-aged women with acute coronary syndrome. Psychoneu-roendocrinology. 2002; 27: 893-906.
- Varma VK, Rushing JT, Ettinger WH. High density lipoprotein cholesterol is associated with serum cortisol in older people. JAGS 1995; 43: 1345-1349.
- Schwertner HA, Troxler RG, Uhl GS, Jackson WG. Relationship between cortisol and cholesterol in men with coronary artery disease and type A behavior. Arte-riosclerosis 1984; 4: 59-64.
- Grad B, Rosenberg GM, Liberman H. Diurnal variation of serum cortisol levels of geriatric subjects. J Gerontol 1971; 26: 351-357.
- Truhan AP, Ahmed R. Corticosteroids: a review with emphasis on complications of prolonged systemic ther-apy. Ann Allergy 1989; 62: 375-390.
- Seeman TE, Robbins RJ. Ageing and hypothalamic-pituitary-adrenal response to challenge in humans. En-docr Rev 1994; 15: 233-260.
- Li XF, Michell JC, Wood S, Coen CW, Lightman SL, O’Byrne KT. The effect of oestradiol and progesterone on hypoglycaemic stress-induced suppression of pulsa-tile luteinizing hormone release and on corticotrophin-releasing hormone mRNA expression in the rat. J Neu-roendocrinol 2003; 15: 468-476.
- Sapolsky RM. Endocrinology alfresco: psychoendocrine studies of wild baboons. Recent Prog Horm Res. 1993; 48:437-468.
- Sapolsky RM. Stress-induced suppression of testicular function in the wild baboon: role of glucocorticoids. En-docrinology. 1985; 116: 2273-2278.
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993; 58: 113-121.
- Mallick J, Stoddart DM, Jones I, Bradley AJ. Behavioral and endocrinological correlates of social status in the male sugar glider. Physiol Behav 1994; 55: 1131-1134.
- Buckingham JC, Cowell A-M, Gillies GE, Herbison AE, Steel JH. The neuroendocrine system: anatomy, physi-ology and responses to stress. In: Buckingham JC, Gillies GE, Cowell A-M. Stress, Stress Hormones and the Immune System. London, UK: John Wiley & Sons; 1997.
- Selhub J, Jaques PF, Wilson P, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in and eldery population. JAMA 1993; 270: 2693-2695.
- Wald DS, Law M, Morris JK Homocysteine and cardio-vascular disease: evidence on causality from a meta-analysis. BMJ 2002; 325: 1202.
- Yadav AS, Bhagwat VR Rathod IM. Relationship of plasma homocysteine with lipid profile parameters in ischemic heart disease. Ind J Clin Biochem 2006, 21 (1): 106-110.
- Smith DG, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, Testosterone, and Coronary Heart Disease Prospective Evidence From the Caerphilly Study. Circulation 2005; 112: 332-340.